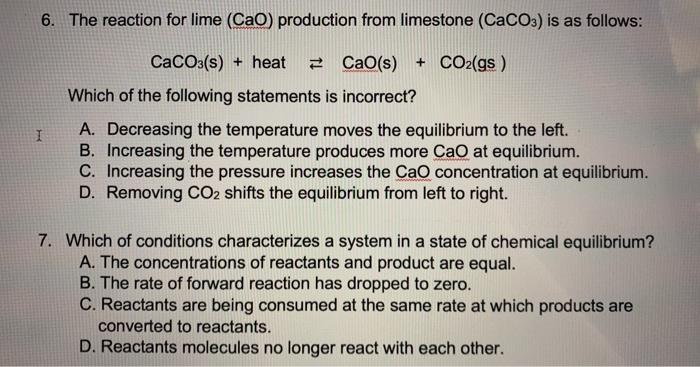
Which Of The Following Conditions Characterizes A System In A State Of Chemical Equilibrium?
Which of the following conditions characterizes a system in a state of chemical equilibrium?. D has zero kinetic energy. In chemical reactions the state in which the conversion of reactants into products and the conversion of products back into reactants occur simultaneously at the same rate. The chemical equilibrium state describes concentrations of reactants and products in a reaction taking place in a closed systemwhich no longer change with time.
Reactants are being consumed at the same rate they are being produced. In other words the rate of the forward reaction equals the rate of the reverse reaction such that the concentrations of reactants and products remain fairly stable in a chemical reaction. Reactants are being consumed at the same rate they are being produced D.
CReactants are being consumed at the same rate they are being produced. Reactant molecules no longer react with each other. Conditions for chemical equilibrium are listed.
A phase equilibrium occurs when a substance is in equilibrium between two states. The concentrations of reactants and products are equal. B releases energy at a steady rate.
AConcentrations of reactants and products are equal. Which of the following conditions characterizes a system in a state of chemical equilibrium. A consumes energy at a steady rate.
A Concentrations of reactants and products are equal. According to the conditions of equilibrium such a transition occurs at constant pressure and temperature. The state of chemical equilibrium is characterized by the constancy of certain properties such as concentration density pressure or color.
A Reactants are being consumed at the same rate they are being produced. Chemical reaction that can proceed in both the forward and reverse directions under given conditions.
The chemical equilibrium is a state in which the rate of the forward reaction is equal to the rate of the reverse reaction.
A phase equilibrium occurs when a substance is in equilibrium between two states. In chemical reactions the state in which the conversion of reactants into products and the conversion of products back into reactants occur simultaneously at the same rate. The chemical equilibrium state describes concentrations of reactants and products in a reaction taking place in a closed systemwhich no longer change with time. Chemical equilibrium is the state of a reversible reaction where the rate of the forward reaction equals the rate of the reverse reaction. Conditions for chemical equilibrium are listed. Which of the following conditions characterizes a system in a state of chemical equilibrium. The chemical equilibrium is a state in which the rate of the forward reaction is equal to the rate of the reverse reaction. E can do no work. Which of the following conditions characterizes a system in a state of chemical equilibrium.
DReactant molecules no longer react with each other. Which of the following conditions characterizes a system in a state of chemical equilibrium. A phase equilibrium occurs when a substance is in equilibrium between two states. The following is true about the chemical equilibrium. Which of the following conditions characterizes a system in a state of chemical equilibrium-Concentrations of reactions and products are equal-Rate of forward reaction has dropped to zero-Reactants are being consumed at the same rate they are being produced-Reactant molecules no loger react with each other. Concentrations of reactants and products are equal. Product concentrations are greater than reactant concentrations.



























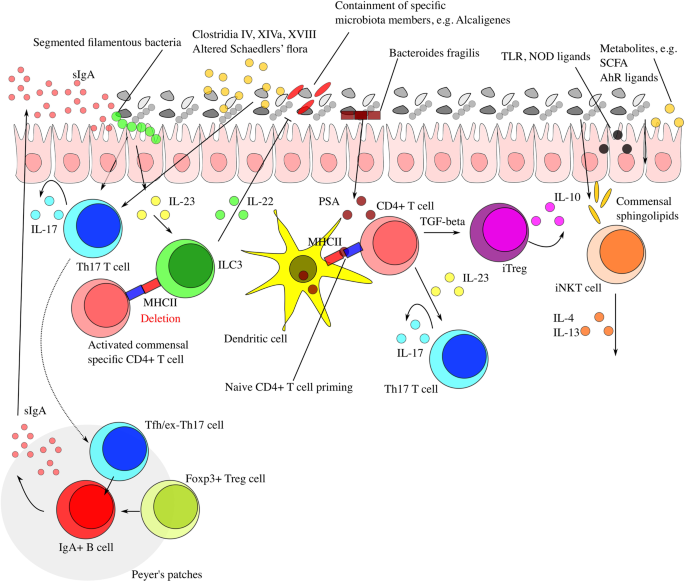
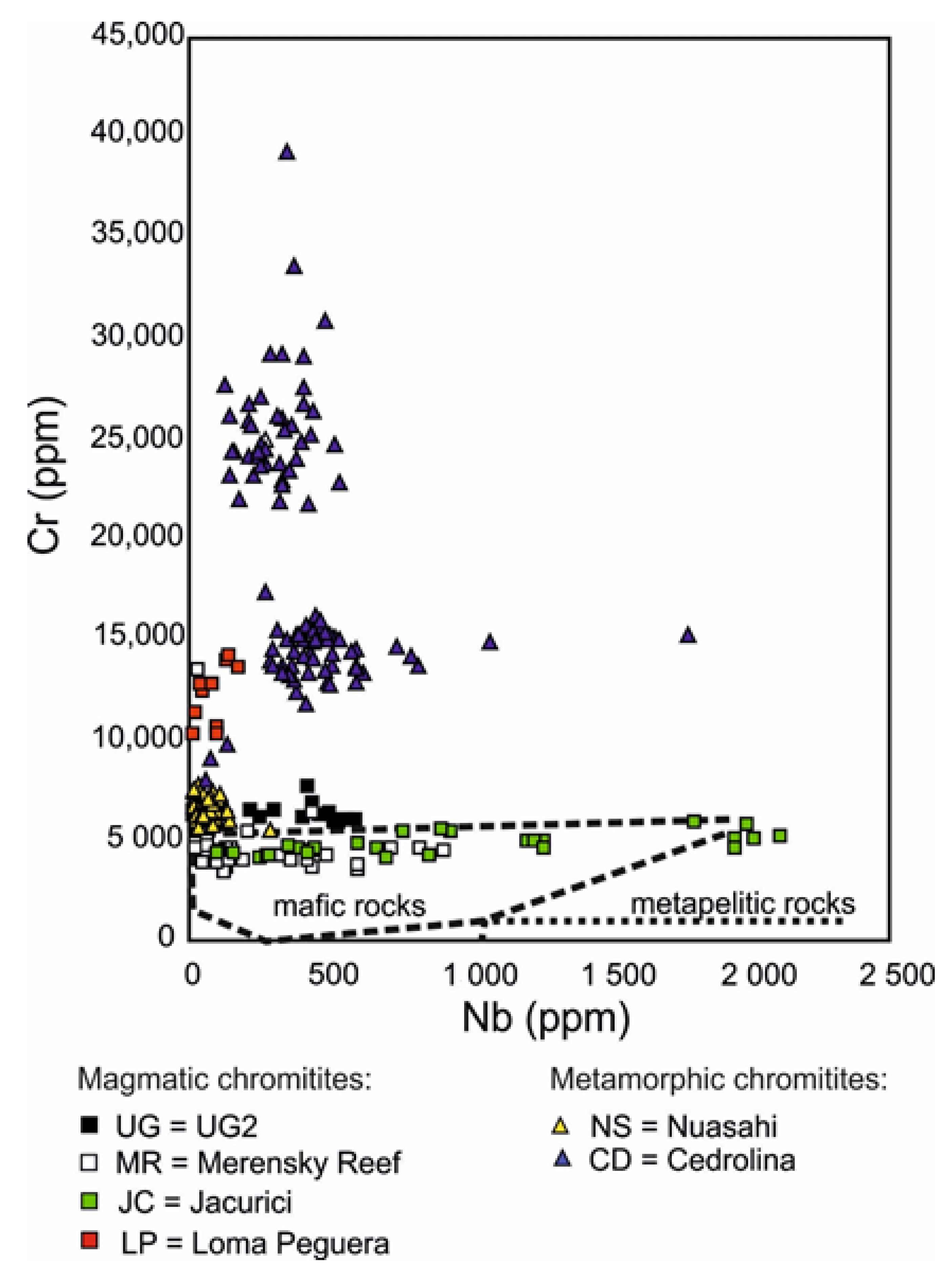


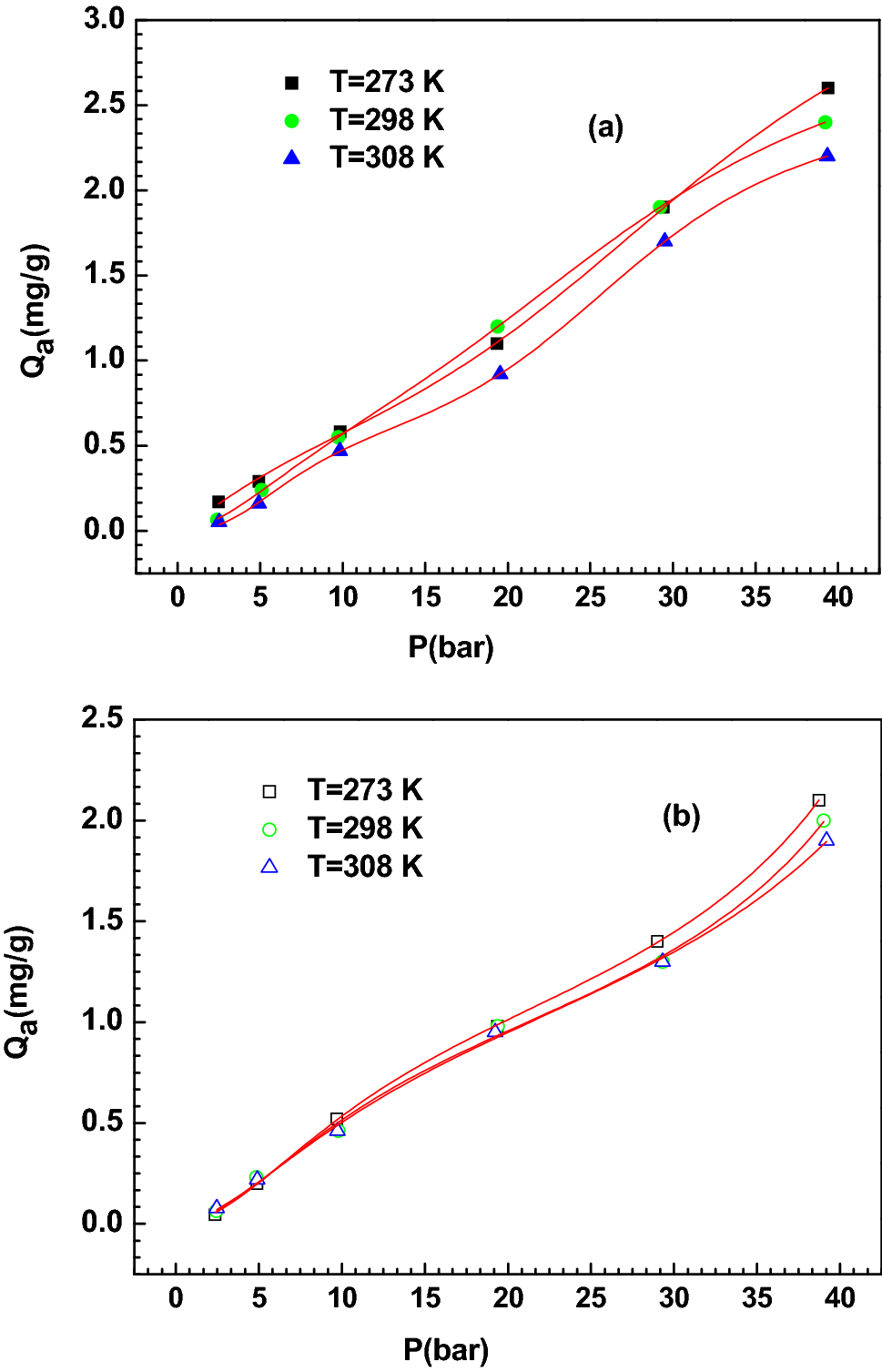

Post a Comment for "Which Of The Following Conditions Characterizes A System In A State Of Chemical Equilibrium?"